When David, a procurement manager at a leading German pharmaceutical company, contacted us last month, he was facing a critical challenge. His facility needed cable glands that could withstand repeated autoclave sterilization cycles without compromising sealing integrity. “Chuck, we’ve had three suppliers fail us already,” he said with evident frustration. “Their glands either crack after a few cycles or lose their IP rating completely.”
Sterilization methods significantly impact cable gland materials, with autoclave sterilization1 causing thermal stress and dimensional changes, while gamma radiation2 can degrade polymer chains and affect mechanical properties. Understanding these effects is crucial for selecting the right materials and ensuring long-term reliability in medical, pharmaceutical, and food processing applications.
This challenge isn’t unique to David’s company. Across the medical device industry, engineers struggle to balance sterilization requirements with material durability. The wrong choice can lead to contamination risks, equipment failures, and costly downtime. Let me share what I’ve learned from 10+ years helping companies navigate these complex material science challenges.
Innholdsfortegnelse
- How Does Autoclave Sterilization Affect Cable Gland Materials?
- What Impact Does Gamma Radiation Have on Gland Components?
- Which Materials Perform Best Under Different Sterilization Methods?
- How Can You Optimize Gland Selection for Sterilization Applications?
- FAQs About Sterilization Effects on Cable Glands
How Does Autoclave Sterilization Affect Cable Gland Materials?
Autoclave sterilization presents unique challenges that many engineers underestimate until it’s too late.
Autoclave sterilization exposes cable glands to temperatures of 121-134°C and pressures up to 2.2 bar, causing thermal expansion, material degradation, and potential seal failure in unsuitable materials.

Thermal Stress and Expansion Effects
The repeated heating and cooling cycles create significant thermal stress within gland components. Different materials expand at different rates, which can compromise the integrity of multi-material assemblies. For instance, standard nylon cable glands may experience:
- Dimensional changes: Up to 2-3% expansion during heating cycles
- Creep deformation: Gradual shape changes under sustained temperature and pressure
- Seal degradation: O-rings and gaskets losing elasticity over multiple cycles
Material-Specific Responses
Nylon 66 Performance: Standard nylon shows good initial resistance but degrades after 50-100 cycles. We’ve observed yellowing, brittleness, and reduced impact strength in field applications.
PEEK Excellence: Polyetheretherketone maintains dimensional stability and chemical resistance through thousands of autoclave cycles. Hassan, who manages a medical device manufacturing facility in Dubai, switched to our PEEK3 cable glands after experiencing failures with standard materials. “The initial cost was higher,” he told me, “but we’ve had zero failures in 18 months of daily sterilization cycles.”
Stainless Steel Reliability: 316L stainless steel bodies provide excellent autoclave resistance, though seal materials remain critical. The thermal conductivity helps maintain uniform temperature distribution, reducing stress concentrations.
Critical Failure Points
The most vulnerable components during autoclave sterilization include:
- Elastomeric seals and O-rings
- Thread interfaces between dissimilar materials
- Cable entry points where multiple materials meet
- Pressure relief mechanisms in sealed enclosures
What Impact Does Gamma Radiation Have on Gland Components?
Gamma sterilization presents entirely different challenges that require specialized material knowledge.
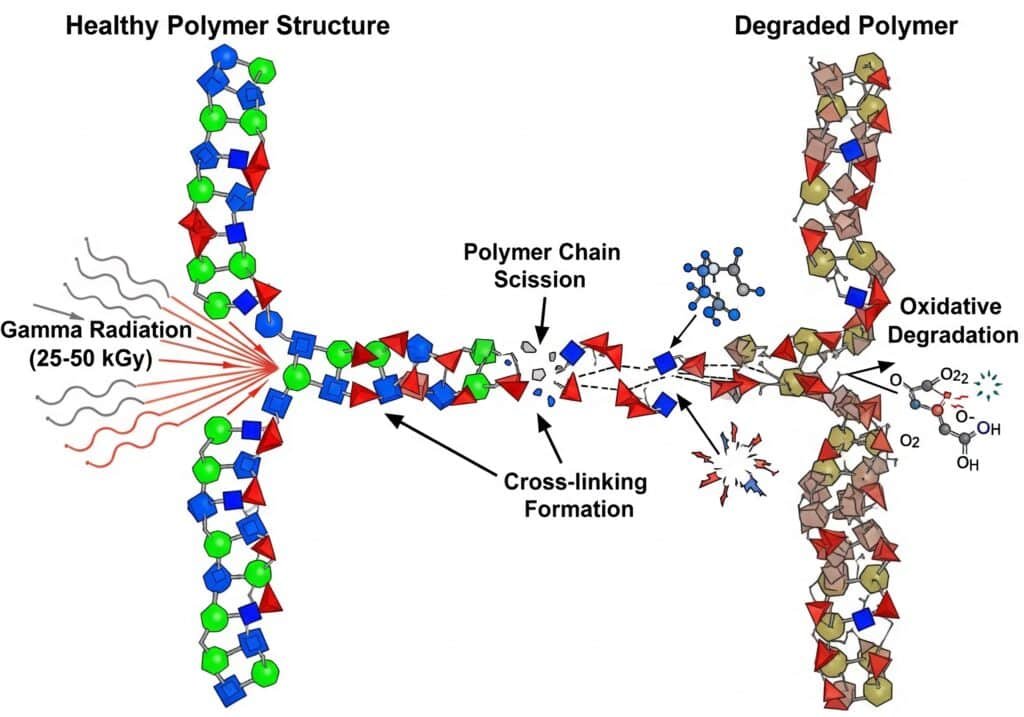
Gamma radiation breaks polymer chains and creates free radicals, leading to embrittlement, discoloration, and loss of mechanical properties in sensitive materials, while having minimal effect on metals and ceramics.
Radiation Dose Effects
Typical gamma sterilization uses 25-50 kGy4 doses, which can cause:
Spalting av polymerkjeder5: High-energy photons break molecular bonds, reducing molecular weight and mechanical strength. This effect is cumulative and irreversible.
Cross-linking Formation: Some polymers form additional cross-links under radiation, potentially improving certain properties while reducing flexibility.
Oksidativ nedbrytning: Radiation creates reactive species that continue degrading materials long after exposure, particularly in oxygen-rich environments.
Sammenligning av materialytelse
| Materiale | Gamma Resistance | Typical Dose Limit | Viktige betraktninger |
|---|---|---|---|
| Nylon 66 | Moderat | 25-50 kGy | Yellowing, embrittlement |
| PEEK | Utmerket | >100 kGy | Minimal property changes |
| PTFE | Dårlig | <25 kGy | Severe degradation |
| 316L SS | Utmerket | No practical limit | Unaffected |
| Silikon | Bra | 50-100 kGy | Some hardening |
Long-term Degradation Patterns
Unlike autoclave effects that appear immediately, gamma radiation damage often manifests over time. We’ve tracked glands in pharmaceutical facilities and found that radiation-induced degradation continues for months after sterilization, particularly affecting:
- Seal compression set resistance
- Thread engagement torque requirements
- Cable grip strength and retention
Which Materials Perform Best Under Different Sterilization Methods?
Selecting the optimal material combination requires understanding both immediate and long-term performance characteristics.
PEEK and 316L stainless steel offer superior performance across both sterilization methods, while specialized fluoropolymers and medical-grade silicones provide excellent seal integrity under specific conditions.
Autoclave-Optimized Materials
Primary Body Materials:
- PEEK: Outstanding thermal stability, minimal creep, excellent chemical resistance
- 316L rustfritt stål: Superior durability, uniform heat distribution, corrosion resistance
- Modified PPS: Good performance at lower cost than PEEK
Sealing Solutions:
- FFKM (perfluorelastomer): Excellent high-temperature performance, chemical inertness
- Medical-grade EPDM: Cost-effective for moderate temperature applications
- PTFE-encapsulated O-rings: Combine PTFE chemical resistance with elastomer sealing
Gamma-Resistant Combinations
For gamma sterilization applications, material selection focuses on radiation stability:
Optimal Configurations:
- Stainless steel bodies with PEEK inserts
- Silicone seals with appropriate hardness ratings
- Ceramic-filled composites for extreme applications
A recent project with a Japanese medical device manufacturer required glands capable of withstanding both sterilization methods. We developed a hybrid solution using 316L stainless steel bodies, PEEK cable grips, and specially formulated FFKM seals. After 500 combined sterilization cycles, all performance parameters remained within specification.
Optimalisering av kostnad og ytelse
While premium materials offer superior performance, cost considerations often drive material selection:
High-Performance Tier: PEEK/316L combinations for critical applications
Mid-Range Solutions: Modified nylon with upgraded seals for moderate duty
Budget Options: Standard nylon with enhanced seal materials for limited cycles
How Can You Optimize Gland Selection for Sterilization Applications?
Successful gland selection requires systematic evaluation of application requirements and sterilization protocols.
Optimize gland selection by analyzing sterilization frequency, temperature/radiation exposure levels, chemical compatibility requirements, and total cost of ownership including replacement and downtime costs.
Rammeverk for vurdering av applikasjoner
Step 1: Sterilization Protocol Analysis
- Document exact temperature, pressure, and time parameters
- Identify radiation dose levels and exposure frequency
- Consider combination sterilization requirements
- Evaluate chemical exposure during and between cycles
Trinn 2: Krav til ytelse
- Define minimum IP rating maintenance
- Specify cable retention force requirements
- Establish acceptable service life expectations
- Identify critical failure consequences
Trinn 3: Økonomisk evaluering
- Calculate total cost of ownership over expected service life
- Include replacement labor costs and downtime expenses
- Consider inventory and spare parts requirements
- Evaluate supplier qualification and certification costs
Designhensyn
Termisk styring: Design assemblies to minimize thermal stress concentrations. Use materials with similar expansion coefficients where possible, and provide stress relief in critical areas.
Seal Design: Implement redundant sealing where critical. Consider dynamic seals for applications with thermal cycling, and static seals for radiation-only applications.
Materialkompatibilitet: Ensure all materials in the assembly are compatible with both the sterilization method and the operating environment. Pay special attention to metal-polymer interfaces.
Validering og testing
Proper validation prevents costly field failures:
- Accelerated aging tests simulating multiple sterilization cycles
- IP rating verification after sterilization exposure
- Mechanical property testing of critical components
- Long-term performance monitoring in actual applications
Konklusjon
The impact of sterilization methods on cable gland materials is complex and application-specific. Autoclave sterilization primarily affects materials through thermal stress and dimensional changes, while gamma radiation causes molecular-level degradation that continues over time. Success requires careful material selection, proper design considerations, and thorough validation testing. Whether you’re dealing with daily autoclave cycles like David’s pharmaceutical facility or combination sterilization requirements, understanding these material interactions is crucial for reliable, long-term performance. 😉
FAQs About Sterilization Effects on Cable Glands
Q: How many autoclave cycles can standard nylon cable glands withstand?
A: Standard nylon 66 cable glands typically withstand 50-100 autoclave cycles before showing significant degradation. Performance varies based on specific temperature, pressure, and cycle duration parameters.
Q: What’s the difference between gamma and autoclave sterilization effects on seals?
A: Autoclave sterilization causes immediate thermal degradation and compression set in seals, while gamma radiation creates long-term molecular damage that continues after exposure. Autoclave effects are predictable and immediate, gamma effects are cumulative and delayed.
Q: Can cable glands be sterilized multiple times with different methods?
A: Yes, but material selection becomes critical. PEEK and 316L stainless steel combinations handle multiple sterilization methods well, while standard nylon and PTFE materials may fail rapidly under combined exposure.
Q: How do I know if my cable glands are suitable for sterilization?
A: Check manufacturer specifications for sterilization compatibility, temperature ratings, and cycle limits. Request test data showing IP rating maintenance after sterilization exposure. When in doubt, conduct qualification testing with your specific sterilization parameters.
Q: What’s the most cost-effective material for moderate sterilization requirements?
A: Modified nylon with upgraded EPDM or silicone seals offers good performance for moderate autoclave requirements (20-50 cycles). For gamma applications, consider nylon with silicone seals as a mid-range solution between standard materials and premium PEEK options.
-
Learn about the principles of steam sterilization and how autoclaves use high-pressure, high-temperature steam to kill microorganisms. ↩
-
Discover how gamma rays are used to sterilize medical devices and other products by breaking down microbial DNA. ↩
-
Explore the exceptional mechanical, thermal, and chemical resistance properties of this high-performance engineering thermoplastic. ↩
-
Understand the definition of the gray (Gy) and kilogray (kGy) as units of absorbed ionizing radiation dose. ↩
-
Learn about the chemical process of chain scission, where polymer chains are broken, leading to a reduction in molecular weight. ↩



