Medical device sterilization failures cost manufacturers millions annually, with 15-20% of failures attributed to inadequate venting that prevents proper sterilant penetration. Standard vent plugs fail under autoclave1 temperatures of 121-134°C or degrade when exposed to ethylene oxide (ETO) sterilization2 chemicals, compromising sterility assurance and regulatory compliance.
Sterilizable vent plugs enable effective sterilization by allowing steam or ETO gas penetration while maintaining sterile barriers post-sterilization. PTFE membrane vents withstand autoclave temperatures up to 150°C and resist ETO chemical degradation, ensuring reliable sterility maintenance for medical devices, pharmaceutical equipment, and laboratory instruments requiring validated sterilization processes.
Last year, I worked with Dr. Sarah Mitchell, quality director at a leading medical device manufacturer in Boston, who was experiencing sterilization validation failures on their implantable device packaging. Their standard nylon vent plugs were melting during autoclave cycles and blocking proper steam penetration. After switching to our pharmaceutical-grade PTFE sterilizable vent plugs with validated temperature resistance, they achieved 100% sterilization efficacy across 1,000 validation cycles – ensuring FDA compliance and patient safety! 🏥
Table of Contents
- What Are Sterilizable Vent Plugs and Why Are They Critical?
- How Do Different Sterilization Methods Affect Vent Plug Materials?
- Which Materials Are Best for Autoclave Sterilization?
- What Materials Work Best for ETO Sterilization Processes?
- How Do You Select and Validate Sterilizable Vent Plugs?
- FAQs About Sterilizable Vent Plugs
What Are Sterilizable Vent Plugs and Why Are They Critical?
Understanding the role of sterilizable vent plugs in medical device and pharmaceutical manufacturing is essential for maintaining sterility assurance and regulatory compliance.
Sterilizable vent plugs are specialized breathable vents designed to withstand sterilization processes while allowing sterilant penetration and maintaining sterile barriers. They enable air displacement during sterilization, prevent vacuum formation during cooling, and maintain sterile conditions post-sterilization. Critical applications include medical device packaging, pharmaceutical containers, laboratory equipment, and sterile processing equipment.
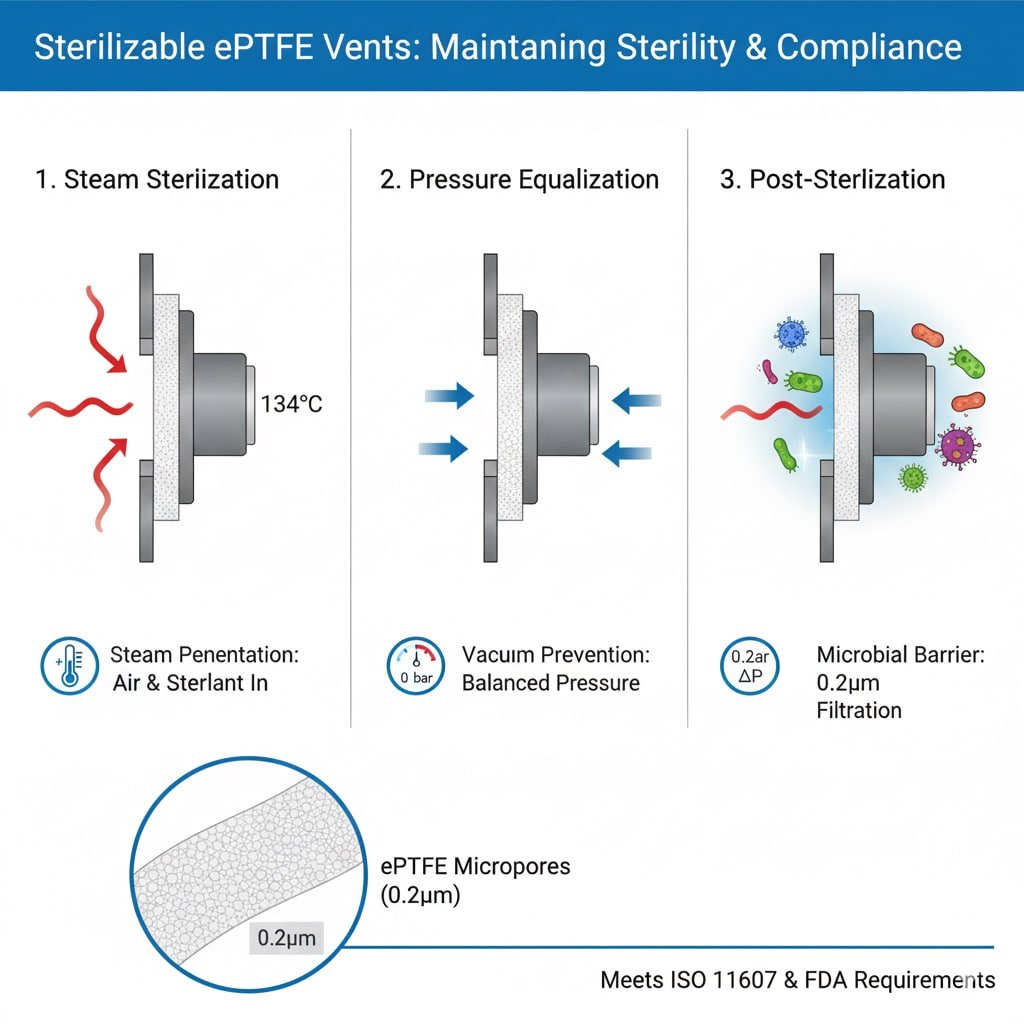
Sterilization Process Requirements
Steam Penetration: Autoclave sterilization requires steam to penetrate packaging and contact all surfaces, necessitating vents that allow gas flow while maintaining microbial barriers.
Air Displacement: Effective sterilization requires complete air removal and replacement with sterilant, which vents facilitate through controlled gas exchange.
Pressure Equalization: Sterilization cycles create pressure differentials that vents must accommodate without compromising sterile barriers or package integrity.
Critical Performance Parameters
Temperature Resistance: Sterilizable vents must maintain structural integrity and filtration performance at sterilization temperatures ranging from 121°C to 150°C.
Chemical Compatibility: Materials must resist degradation from sterilization chemicals including steam, ethylene oxide, hydrogen peroxide, and ozone.
Microbial Barrier Efficiency: Post-sterilization, vents must provide a reliable microbial barrier3 with pore sizes typically 0.2-0.22 microns for bacterial retention.
Regulatory Compliance Considerations
FDA Validation Requirements: Medical device applications require validated sterilization processes with documented vent performance throughout sterilization cycles.
ISO Standards Compliance: Sterilizable vents must meet ISO 116074 packaging standards and ISO 17665 steam sterilization requirements for medical applications.
Biocompatibility Testing: Medical device contact applications may require USP Class VI biocompatibility testing for patient safety assurance.
Application Categories
Medical Device Packaging: Sterile packaging for implants, surgical instruments, and disposable medical devices requiring sterility maintenance until use.
Pharmaceutical Manufacturing: Sterile processing equipment, bioreactors, and pharmaceutical containers requiring validated sterilization processes.
Laboratory Equipment: Autoclavable laboratory containers, culture vessels, and analytical equipment requiring sterile conditions for accurate results.
How Do Different Sterilization Methods Affect Vent Plug Materials?
Different sterilization methods create unique challenges for vent plug materials, requiring specific material properties and design considerations for optimal performance.
Steam sterilization subjects materials to high temperature (121-134°C) and saturated steam conditions that can cause thermal degradation, dimensional changes, and membrane damage. ETO sterilization exposes materials to reactive chemicals at lower temperatures (37-63°C) but longer exposure times that can cause chemical degradation and outgassing. Each method requires specific material selection for reliable performance.
Steam Sterilization Effects
Thermal Stress: High temperatures cause thermal expansion, potential melting of thermoplastic components, and degradation of temperature-sensitive materials.
Hydrolysis Reactions5: Steam exposure can cause hydrolytic degradation of certain polymers, particularly polyesters and some polyamides.
Dimensional Stability: Repeated thermal cycling can cause dimensional changes that affect sealing performance and filtration efficiency.
ETO Sterilization Challenges
Chemical Reactivity: Ethylene oxide reacts with materials containing active hydrogen atoms, potentially altering material properties and creating toxic residues.
Outgassing Requirements: ETO-sterilized products require extended aeration periods to remove absorbed ETO and reaction products before safe use.
Penetration Characteristics: ETO requires specific humidity and temperature conditions for effective penetration, affecting vent design requirements.
Hydrogen Peroxide Plasma Effects
Oxidative Degradation: H2O2 plasma creates highly reactive species that can degrade organic materials through oxidation reactions.
Material Compatibility: Many elastomers and some plastics are incompatible with H2O2 plasma sterilization due to rapid degradation.
Low Temperature Benefits: Plasma sterilization operates at low temperatures (45-55°C), reducing thermal stress on temperature-sensitive materials.
Gamma Radiation Considerations
Radiation Damage: High-energy gamma radiation can cause polymer chain scission or cross-linking, altering material properties significantly.
Dose Accumulation: Repeated gamma sterilization can cause cumulative damage, limiting the number of sterilization cycles materials can withstand.
Antioxidant Requirements: Radiation-resistant formulations often require antioxidants to prevent oxidative degradation during and after irradiation.
I recently helped Ahmed Al-Rashid, operations manager at a pharmaceutical facility in Dubai, solve ETO sterilization validation issues with their bioreactor venting systems. Their standard vent plugs were absorbing ETO and requiring extended 14-day aeration periods that disrupted production schedules. By implementing our ETO-compatible PTFE vent plugs with minimal absorption characteristics, they reduced aeration time to 24 hours while maintaining full sterility assurance – dramatically improving production efficiency! 🚀
Which Materials Are Best for Autoclave Sterilization?
Selecting appropriate materials for autoclave sterilization requires understanding thermal stability, hydrolysis resistance, and long-term performance under repeated steam exposure.
PTFE (polytetrafluoroethylene) offers superior autoclave performance with continuous service temperatures to 260°C, excellent chemical inertness, and hydrolysis resistance. PVDF (polyvinylidene fluoride) provides good thermal stability to 150°C with lower cost. Avoid nylon, standard polyethylene, and most elastomers that degrade under autoclave conditions, compromising filtration and sealing performance.
PTFE Membrane Advantages
Exceptional Temperature Resistance: PTFE maintains structural integrity and filtration performance at temperatures well above typical autoclave conditions (121-134°C).
Chemical Inertness: PTFE resists degradation from steam, cleaning chemicals, and sterilization byproducts, ensuring consistent long-term performance.
Hydrophobic Properties: PTFE’s hydrophobic nature prevents water absorption and maintains dimensional stability throughout sterilization cycles.
Material Performance Comparison
| Material | Max Temp (°C) | Steam Resistance | Hydrolysis Resistance | Cost Factor |
|---|---|---|---|---|
| PTFE | 260 | Excellent | Excellent | High |
| PVDF | 150 | Good | Good | Medium |
| PP (Polypropylene) | 135 | Fair | Fair | Low |
| Nylon | 80-100 | Poor | Poor | Low |
Housing Material Selection
Stainless Steel 316L: Provides excellent corrosion resistance, thermal stability, and cleanability for pharmaceutical and medical applications requiring validated cleaning procedures.
PEEK (Polyetheretherketone): Offers outstanding thermal stability (continuous use to 250°C) with excellent chemical resistance for demanding autoclave applications.
Polypropylene: Cost-effective option for single-use applications with adequate performance for standard autoclave cycles at 121°C.
Sealing Component Considerations
EPDM O-Rings: Provide good steam resistance and thermal stability for temperatures up to 150°C with excellent sealing performance.
Silicone Seals: Offer superior temperature resistance (up to 200°C) but may have compatibility issues with certain cleaning chemicals.
PTFE Encapsulated O-Rings: Combine PTFE chemical resistance with elastomer sealing properties for demanding applications requiring both performance characteristics.
Design Optimization for Autoclave Use
Thermal Expansion Accommodation: Vent designs must accommodate differential thermal expansion between materials to prevent seal failure during temperature cycling.
Drainage Features: Proper drainage design prevents condensate accumulation that could compromise filtration performance or create contamination risks.
Validation Support: Design features should facilitate validation testing including pressure decay, microbial challenge, and thermal performance verification.
What Materials Work Best for ETO Sterilization Processes?
ETO sterilization presents unique material challenges requiring chemical compatibility, minimal absorption, and rapid outgassing characteristics for efficient processing.
PTFE and PVDF offer excellent ETO compatibility with minimal chemical absorption and rapid outgassing. Avoid materials with active hydrogen sites like nylon, PVC, and natural rubber that react with ETO forming toxic compounds. Stainless steel housings provide optimal chemical resistance, while silicone seals offer good ETO compatibility with acceptable outgassing characteristics for most applications.
ETO Chemical Compatibility
Reaction Mechanisms: ETO reacts with materials containing hydroxyl, amino, carboxyl, and sulfhydryl groups, forming ethylene glycol derivatives and other potentially toxic compounds.
Absorption Characteristics: Materials with high ETO absorption require extended aeration periods, increasing processing time and costs significantly.
Outgassing Kinetics: Rapid outgassing materials enable shorter aeration cycles, improving process efficiency and reducing inventory holding times.
Material ETO Performance Rankings
Excellent ETO Compatibility:
- PTFE: Minimal absorption, rapid outgassing, no chemical reactivity
- PVDF: Low absorption, good outgassing, excellent chemical resistance
- Stainless Steel: No absorption, immediate use capability
Good ETO Compatibility:
- Polypropylene: Moderate absorption, acceptable outgassing
- Silicone: Low reactivity, moderate outgassing requirements
Poor ETO Compatibility:
- Nylon: High reactivity, extended aeration required
- PVC: Chemical degradation, toxic compound formation
- Natural Rubber: High absorption, potential degradation
Aeration Time Requirements
| Material | Typical Aeration Time | ETO Absorption Level | Outgassing Rate |
|---|---|---|---|
| PTFE | 8-24 hours | Minimal | Rapid |
| PVDF | 24-48 hours | Low | Good |
| Polypropylene | 48-72 hours | Moderate | Moderate |
| Nylon | 7-14 days | High | Slow |
Process Parameter Optimization
Temperature Control: ETO sterilization typically operates at 37-63°C, requiring materials that maintain performance across this temperature range.
Humidity Requirements: ETO effectiveness requires 40-80% relative humidity, necessitating materials that perform consistently under these moisture conditions.
Gas Concentration Management: ETO concentrations of 450-1200 mg/L require materials that resist chemical attack while allowing sterilant penetration.
Validation Considerations
Residue Testing: ETO-sterilized products require testing for ETO residues and reaction products to ensure safety limits compliance.
Biocompatibility Maintenance: Materials must maintain biocompatibility after ETO exposure and aeration, requiring validated material selection.
Process Monitoring: ETO sterilization requires continuous monitoring of temperature, humidity, pressure, and gas concentration throughout the cycle.
How Do You Select and Validate Sterilizable Vent Plugs?
Proper selection and validation of sterilizable vent plugs ensures reliable sterilization performance, regulatory compliance, and long-term operational success.
Selection requires matching material properties to sterilization method, defining performance requirements, and considering regulatory standards. Validation involves thermal performance testing, microbial challenge studies, chemical compatibility assessment, and long-term stability evaluation. Document all testing according to FDA and ISO standards for regulatory submission and quality system compliance.
Selection Criteria Framework
Sterilization Method Compatibility: Match vent materials to specific sterilization methods (steam, ETO, H2O2 plasma, gamma) based on temperature, chemical, and radiation resistance requirements.
Performance Specifications: Define required flow rates, pressure ratings, filtration efficiency, and microbial barrier properties based on application requirements.
Regulatory Requirements: Consider FDA device classification, ISO standard compliance, and biocompatibility requirements for intended use applications.
Application Assessment Parameters
Operating Environment: Evaluate temperature ranges, chemical exposure, pressure conditions, and contamination risks throughout the product lifecycle.
Sterilization Frequency: Consider single-use versus multiple sterilization cycles and cumulative effects on material performance and reliability.
Validation Scope: Determine testing requirements based on risk assessment, regulatory pathway, and quality system requirements.
Validation Testing Protocol
Thermal Performance Testing:
- Temperature cycling at sterilization conditions
- Dimensional stability measurement
- Filtration efficiency verification post-thermal exposure
Microbial Challenge Testing:
- Bacterial challenge with appropriate test organisms
- Sterility maintenance verification
- Long-term barrier integrity assessment
Chemical Compatibility Assessment:
- Material degradation evaluation
- Extractables and leachables testing
- Biocompatibility maintenance verification
Documentation Requirements
Material Specifications: Complete material data sheets including chemical composition, thermal properties, and regulatory certifications.
Test Protocols: Detailed validation protocols following FDA guidance and ISO standards for sterilization validation.
Performance Data: Comprehensive test results demonstrating performance throughout specified operating conditions and sterilization cycles.
Quality System Integration
Supplier Qualification: Establish supplier quality agreements including material traceability, change control, and quality documentation requirements.
Incoming Inspection: Develop inspection procedures for critical dimensions, material properties, and performance characteristics verification.
Process Validation: Integrate vent performance into overall sterilization process validation including worst-case scenario testing.
Risk Management Considerations
Failure Mode Analysis: Identify potential failure modes including material degradation, seal failure, and filtration compromise with appropriate mitigation strategies.
Change Control: Establish procedures for managing material changes, supplier changes, and specification modifications with appropriate revalidation requirements.
Continuous Monitoring: Implement ongoing monitoring programs to verify continued performance and identify potential issues before they affect product quality.
Conclusion
Sterilizable vent plugs play a critical role in ensuring effective sterilization while maintaining sterile barriers in medical device and pharmaceutical applications. Understanding the unique challenges of different sterilization methods and selecting appropriate materials is essential for reliable performance and regulatory compliance.
PTFE-based vent plugs offer superior performance across multiple sterilization methods, providing excellent temperature resistance, chemical compatibility, and long-term reliability. Proper selection and validation ensure optimal sterilization effectiveness while minimizing processing time and costs.
At Bepto, our comprehensive range of sterilizable vent plugs includes pharmaceutical-grade PTFE membranes, validated temperature performance, and complete documentation packages for regulatory submissions. With over a decade of experience in specialized venting applications and ISO-certified manufacturing capabilities, we provide the reliable, cost-effective solutions you need for critical sterilization applications. Trust us to keep your sterilization processes validated and your products safe! 🔬
FAQs About Sterilizable Vent Plugs
Q: Can the same vent plug be used for both autoclave and ETO sterilization?
A: Yes, PTFE membrane vent plugs can handle both autoclave and ETO sterilization effectively. PTFE offers excellent temperature resistance for autoclave cycles and minimal ETO absorption for rapid aeration, making it ideal for facilities using multiple sterilization methods.
Q: How many sterilization cycles can a vent plug withstand?
A: High-quality PTFE vent plugs typically withstand 100+ autoclave cycles or 50+ ETO cycles while maintaining filtration performance. Actual cycle life depends on sterilization parameters, handling procedures, and performance acceptance criteria for your specific application.
Q: What pore size is required for sterile filtration in medical applications?
A: Medical applications typically require 0.2 or 0.22 micron pore sizes for reliable bacterial retention. This pore size provides validated sterility assurance while allowing adequate gas flow for effective sterilization and pressure equalization.
Q: Do sterilizable vent plugs require special validation testing?
A: Yes, sterilizable vent plugs require validation testing including thermal performance, microbial challenge, and material compatibility studies. Testing must follow FDA guidance and ISO standards, with documentation supporting your sterilization process validation and regulatory submissions.
Q: How do you prevent vent plug contamination during sterilization?
A: Prevent contamination through proper installation, protective covers during handling, validated sterilization parameters, and appropriate post-sterilization storage. Use sterile technique during installation and ensure vent plugs are designed for your specific sterilization method and application requirements.
-
Learn the scientific principles of autoclave sterilization and how saturated steam under pressure is used to effectively kill microorganisms. ↩
-
Explore the chemical process of ethylene oxide (ETO) sterilization, a low-temperature method used for sterilizing heat- and moisture-sensitive medical devices. ↩
-
Discover the methods used to test and validate the effectiveness of a microbial barrier, ensuring it prevents the ingress of microorganisms and maintains sterility. ↩
-
Review the key requirements of the ISO 11607 standard, which specifies the materials and testing for sterile barrier systems for medical devices. ↩
-
Understand the chemical reaction of hydrolysis and how it can cause the degradation of certain polymer materials when exposed to water or steam at high temperatures. ↩



