Introduction
Brass cable glands fail prematurely in corrosive environments when inadequate plating thickness allows moisture and chemicals to penetrate protective coatings, leading to dezincification1, stress corrosion cracking2, and catastrophic seal failures that can compromise entire electrical systems within months of installation.
Nickel plating thickness of 10-25 microns provides optimal corrosion protection for brass cable glands, with 10 microns suitable for indoor applications, 15 microns for standard marine environments, and 25 microns for severe chemical exposure, delivering 5-10x longer service life compared to unplated brass components.
After a decade of investigating premature brass cable gland failures across industries from offshore oil platforms to chemical processing plants, I’ve learned that plating thickness isn’t just about surface protection—it’s about ensuring long-term reliability in increasingly corrosive operating environments where failure isn’t an option.
Table of Contents
- What Causes Corrosion in Brass Cable Glands?
- How Does Plating Thickness Affect Corrosion Protection?
- Which Plating Materials Offer the Best Corrosion Resistance?
- What Are the Optimal Plating Thickness Requirements for Different Environments?
- How Can You Test and Verify Plating Quality?
- FAQs About Brass Cable Gland Plating and Corrosion
What Causes Corrosion in Brass Cable Glands?
Understanding corrosion mechanisms is essential for selecting appropriate plating specifications and thickness requirements.
Brass cable glands suffer from dezincification, galvanic corrosion3, and stress corrosion cracking when exposed to moisture, chlorides, and acidic environments, with corrosion rates accelerating exponentially above 40°C temperature and 3.5% salt concentration, making protective plating critical for service life extension.
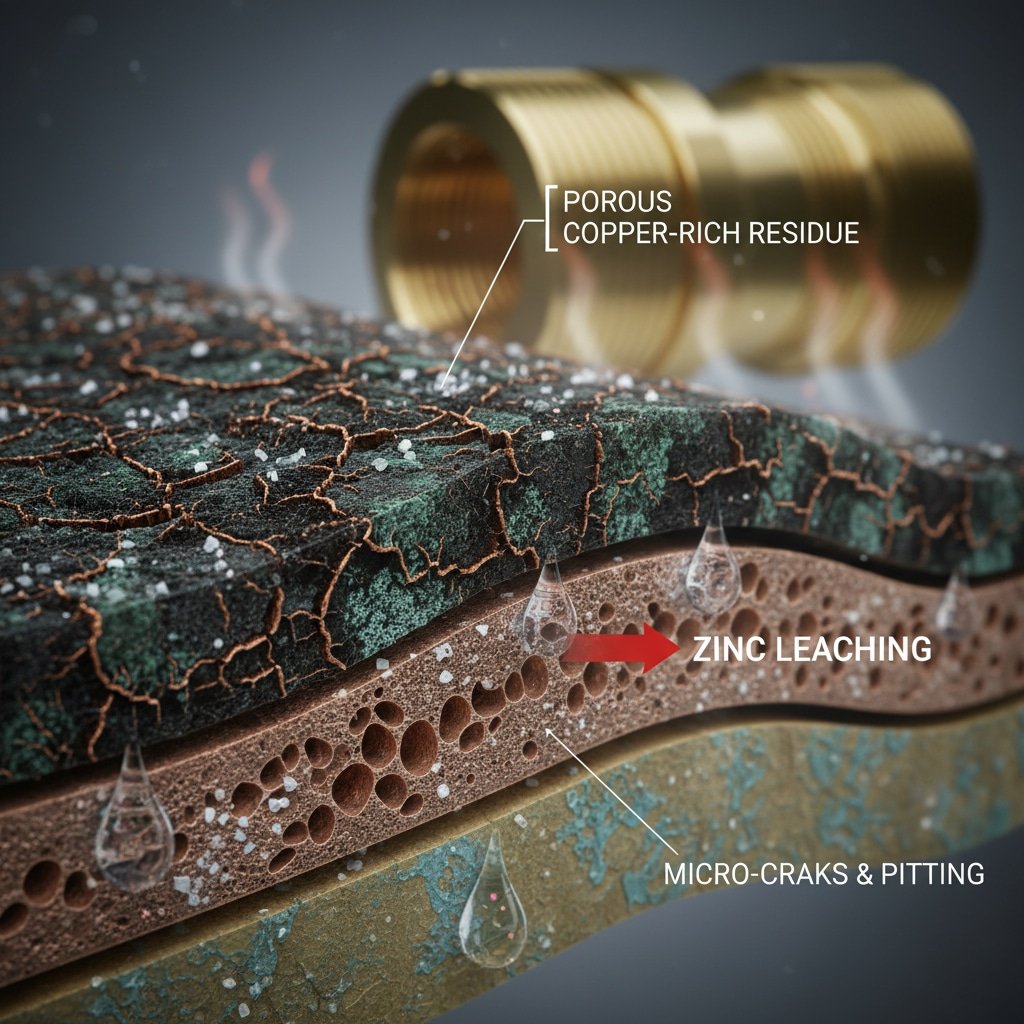
Primary Corrosion Mechanisms
Dezincification Process:
- Selective leaching of zinc from brass alloy
- Leaves porous copper-rich residue
- Dramatically reduces mechanical strength
- Creates pathways for further corrosion
Galvanic Corrosion:
- Occurs when brass contacts dissimilar metals
- Accelerated in presence of electrolytes
- Brass acts as anode in most couples
- Rate depends on area ratio and conductivity
I worked with Henrik, a maintenance manager at a North Sea oil platform off the coast of Norway, where unplated brass cable glands were failing within 18 months due to severe marine exposure. The combination of salt spray, temperature cycling, and hydrogen sulfide created the perfect storm for accelerated corrosion.
Environmental Factors
Chloride Exposure:
- Seawater contains 19,000 ppm chlorides
- Industrial atmospheres: 10-1000 ppm
- Accelerates all corrosion mechanisms
- Penetrates through coating defects
Temperature Effects:
- Corrosion rate doubles every 10°C increase
- Thermal cycling creates stress concentrations
- Expansion/contraction damages coatings
- High temperatures reduce coating adhesion
pH Conditions:
- Acidic environments (pH < 7) accelerate attack
- Alkaline conditions can cause stress cracking
- Neutral pH with chlorides still problematic
- Buffering capacity affects corrosion rate
Henrik’s platform required a comprehensive approach combining optimal plating thickness with environmental sealing to achieve reliable long-term performance in the harsh offshore environment.
Failure Mode Analysis
Coating Breakdown:
- Pinhole formation allows electrolyte penetration
- Coating delamination exposes substrate
- Galvanic cells form at defect sites
- Localized corrosion accelerates failure
Mechanical Degradation:
- Thread engagement loss due to corrosion
- Seal compression reduction from material loss
- Dimensional changes affect cable grip
- Structural integrity compromised
Performance Impact:
- IP rating degradation from seal failure
- Electrical continuity loss in EMC applications
- Cable retention force reduction
- Complete assembly failure possible
How Does Plating Thickness Affect Corrosion Protection?
Plating thickness directly determines the barrier protection and service life of brass cable glands in corrosive environments.
Plating thickness provides barrier protection proportional to coating depth, with each 5 microns of nickel plating extending service life by 2-3 years in marine environments, while insufficient thickness below 8 microns allows rapid penetration and substrate attack within 6-12 months of exposure.
Thickness-Performance Relationship
Barrier Protection Mechanism:
- Physical barrier prevents electrolyte contact
- Thickness determines penetration time
- Defect density inversely related to thickness
- Uniform coverage critical for effectiveness
Service Life Correlation:
| Plating Thickness | Indoor Service Life | Marine Service Life | Chemical Service Life |
|---|---|---|---|
| 5 microns | 3-5 years | 1-2 years | 6-12 months |
| 10 microns | 8-12 years | 3-5 years | 2-3 years |
| 15 microns | 15-20 years | 8-12 years | 5-8 years |
| 25 microns | 25+ years | 15-20 years | 10-15 years |
Economic Optimization:
- Initial cost increases linearly with thickness
- Service life increases exponentially
- Optimal thickness balances cost and performance
- Replacement costs often exceed plating premiums
Coating Integrity Factors
I remember working with Fatima, who manages a petrochemical facility in Jubail, Saudi Arabia, where high-temperature hydrogen sulfide exposure was causing rapid coating failure on standard plated cable glands.
Adhesion Requirements:
- Proper surface preparation essential
- Substrate cleanliness affects bond strength
- Intermediate layers improve adhesion
- Thermal expansion compatibility important
Uniformity Considerations:
- Thickness variation affects local protection
- Complex geometries require special attention
- Current density distribution in plating bath
- Masking and fixturing influence uniformity
Quality Control Measures:
- Thickness measurement at critical points
- Adhesion testing per ASTM standards
- Porosity evaluation methods
- Statistical process control implementation
Fatima’s facility required 20-micron nickel plating with chromium topcoat to achieve reliable performance in their severe chemical environment, extending service life from 18 months to over 8 years.
Which Plating Materials Offer the Best Corrosion Resistance?
Different plating materials provide varying levels of corrosion protection and cost-effectiveness for brass cable glands.
Nickel plating offers the best balance of corrosion resistance and cost-effectiveness for brass cable glands, providing superior barrier protection compared to zinc (3x better) and chrome (2x better), while precious metal plating offers ultimate protection at 10x the cost for critical applications.
Plating Material Comparison
Nickel Plating:
- Excellent corrosion resistance
- Good adhesion to brass substrates
- Moderate cost increase
- Wide temperature range capability
- Standard industrial acceptance
Chrome Plating:
- Superior hardness and wear resistance
- Good chemical resistance
- Higher cost than nickel
- Potential environmental concerns
- Excellent appearance retention
Zinc Plating:
- Sacrificial protection mechanism
- Lower cost option
- Limited service life in marine environments
- Good for mild atmospheric exposure
- Easy processing and repair
Advanced Plating Systems
Multi-Layer Coatings:
- Copper strike for adhesion
- Nickel barrier layer for protection
- Chrome topcoat for durability
- Optimized thickness distribution
Alloy Plating Options:
- Nickel-phosphorus for uniform thickness
- Nickel-tungsten for enhanced hardness
- Zinc-nickel for improved corrosion resistance
- Custom alloys for specific environments
Performance Characteristics:
| Plating Material | Corrosion Resistance | Cost Factor | Temperature Limit | Applications |
|---|---|---|---|---|
| Zinc | Fair | 1.0x | 100°C | Indoor, mild environments |
| Nickel | Excellent | 1.5x | 200°C | General purpose, marine |
| Chrome | Very Good | 2.0x | 250°C | Chemical, high wear |
| Precious Metals | Superior | 10x | 300°C | Critical, aerospace |
At Bepto, we offer multiple plating options to match your specific environmental requirements and budget constraints, ensuring optimal performance and cost-effectiveness for your application.
What Are the Optimal Plating Thickness Requirements for Different Environments?
Environmental conditions dictate minimum plating thickness requirements for reliable long-term performance.
Indoor applications require 8-12 microns nickel plating, marine environments need 15-20 microns, and severe chemical exposure demands 20-25 microns, with thickness selection based on chloride concentration, temperature, and required service life to ensure cost-effective protection.
Environment-Specific Requirements
Indoor/Controlled Environments:
- Temperature: 15-35°C
- Humidity: 30-70% RH
- Chloride exposure: <10 ppm
- Recommended thickness: 8-12 microns
- Expected service life: 15-25 years
Marine/Coastal Applications:
- Salt spray exposure
- Temperature cycling: -10 to +60°C
- Chloride concentration: 100-19,000 ppm
- Recommended thickness: 15-20 microns
- Expected service life: 10-15 years
Chemical Processing:
- Acidic/alkaline exposure
- Temperature: up to 120°C
- Various chemical concentrations
- Recommended thickness: 20-25 microns
- Expected service life: 8-12 years
Selection Methodology
Risk Assessment Factors:
- Failure consequence severity
- Maintenance accessibility
- Replacement cost considerations
- Safety and regulatory requirements
Economic Analysis:
- Initial plating cost premium
- Expected service life extension
- Maintenance and replacement costs
- Total cost of ownership calculation
Quality Specifications:
- Minimum thickness requirements
- Uniformity tolerances
- Adhesion test requirements
- Acceptance criteria definition
I worked with James, a project manager for a wind farm installation off the coast of Scotland, where extreme marine conditions required careful plating specification to ensure 20-year service life for offshore cable glands.
James’s project specified 18-micron nickel plating with strict quality control requirements, resulting in zero corrosion-related failures after five years of operation in the harsh North Atlantic environment.
How Can You Test and Verify Plating Quality?
Comprehensive testing ensures plating thickness and quality meet specification requirements for reliable corrosion protection.
ASTM B5684 magnetic thickness measurement and ASTM B571 adhesion testing provide quantitative verification of plating quality, with salt spray testing per ASTM B1175 validating corrosion resistance performance over 96-1000 hours depending on service requirements.
Thickness Measurement Methods
Magnetic Induction Testing:
- Non-destructive measurement
- Suitable for nickel on brass
- ±1 micron accuracy achievable
- Rapid production testing capability
Eddy Current Testing:
- Non-magnetic coatings on conductive substrates
- Good for complex geometries
- Calibration critical for accuracy
- Portable instrument availability
Microscopic Cross-Section:
- Destructive but highly accurate
- Reveals coating structure and uniformity
- Identifies interface quality
- Required for specification verification
Quality Verification Protocols
Adhesion Testing:
- Bend test per ASTM B571
- Thermal shock evaluation
- Tape test for coating integrity
- Scratch test for bond strength
Corrosion Testing:
- Salt spray per ASTM B117
- Cyclic corrosion testing
- Electrochemical evaluation
- Accelerated aging protocols
Statistical Sampling:
- Production lot verification
- Critical dimension focus
- Statistical process control
- Supplier qualification requirements
Production Quality Control
Incoming Material Verification:
- Substrate composition analysis
- Surface preparation validation
- Cleanliness assessment
- Dimensional accuracy check
Process Monitoring:
- Bath composition control
- Current density optimization
- Temperature and time tracking
- Thickness measurement frequency
Final Inspection:
- 100% thickness verification at critical points
- Visual inspection for defects
- Adhesion testing on sample basis
- Documentation and traceability
Our quality laboratory at Bepto maintains comprehensive testing capabilities to ensure all plated cable glands meet or exceed specification requirements, providing documented verification of corrosion protection performance.
Conclusion
Plating thickness is the critical factor determining corrosion resistance and service life of brass cable glands in demanding environments. While thicker plating increases initial cost, the exponential improvement in service life makes it highly cost-effective for most applications. Nickel plating at 10-25 microns provides optimal protection, with thickness selection based on environmental severity and required service life. Indoor applications can use 8-12 microns, marine environments require 15-20 microns, and chemical exposure demands 20-25 microns for reliable long-term performance. At Bepto, we combine extensive testing capabilities with practical application experience to help you select the optimal plating specification for your brass cable gland requirements. Remember, investing in proper plating thickness today prevents costly corrosion failures and system downtime tomorrow! 😉
FAQs About Brass Cable Gland Plating and Corrosion
Q: What plating thickness do I need for marine cable glands?
A: Marine applications require 15-20 microns of nickel plating for reliable corrosion protection. This thickness provides 10-15 years service life in salt spray environments compared to 1-2 years for unplated brass components.
Q: How can I tell if my brass cable glands have adequate plating thickness?
A: Use magnetic thickness gauges for non-destructive measurement of nickel plating on brass. Minimum 8 microns for indoor use, 15 microns for marine, and 20 microns for chemical environments are recommended specifications.
Q: Does thicker plating always provide better corrosion protection?
A: Yes, up to practical limits. Each additional 5 microns of nickel plating typically doubles service life in corrosive environments. However, beyond 25 microns, cost increases faster than performance benefits for most applications.
Q: Can I repair damaged plating on brass cable glands?
A: Minor damage can be repaired with cold galvanizing compounds or brush plating, but complete replating is recommended for critical applications. Localized repairs may create galvanic corrosion cells that accelerate failure.
Q: How do I verify plating quality from suppliers?
A: Request certificates showing thickness measurements per ASTM B568, adhesion test results per ASTM B571, and salt spray test data per ASTM B117. Verify measurements at multiple points on sample components before approving production lots.
-
Understand the metallurgical process of dezincification, where zinc is selectively leached from brass alloys, leaving a weakened copper structure. ↩
-
Learn about the failure mechanism of Stress Corrosion Cracking (SCC), which results from the combined influence of tensile stress and a corrosive environment. ↩
-
Explore the electrochemical principles of galvanic corrosion and review the galvanic series to see how different metals interact in an electrolyte. ↩
-
Review the official ASTM B568 standard for measuring coating thickness using X-ray spectrometry, a common non-destructive testing method. ↩
-
Access the details of the ASTM B117 standard, the industry-wide accepted practice for operating salt spray (fog) apparatus for corrosion testing. ↩



