A single non-compliant cable gland can trigger FDA violations costing millions in recalls and shutdowns. Food processing plants need specialized solutions that meet strict regulatory requirements.
Food grade cable glands require FDA-approved materials (21 CFR 177), NSF certification for direct food contact applications, proper sanitary design principles, chemical resistance to cleaning agents, and documentation supporting HACCP compliance1 for safe food processing operations.
Last week, David called me in panic. His dairy processing plant faced an FDA inspection, and they discovered several cable glands in their clean rooms weren’t food grade compliant. The potential violations could have shut down production worth €2 million per day. This incident highlights why food grade compliance isn’t optional in processing plants.
Table of Contents
- What Are the Essential Food Grade Requirements for Cable Glands?
- How Do FDA and NSF Standards Apply to Cable Gland Materials and Design?
- What Sanitary Design Principles Must Cable Glands Follow in Food Processing?
- How Do You Specify and Verify Food Grade Cable Glands for Different Processing Zones?
What Are the Essential Food Grade Requirements for Cable Glands?
Food grade cable glands must meet stringent material, design, and documentation requirements that go far beyond standard industrial applications. Understanding these requirements prevents costly compliance failures.
Essential food grade requirements include FDA-compliant materials per 21 CFR 177.2600, NSF/ANSI 51 certification for food equipment, sanitary design with smooth surfaces and minimal crevices, resistance to cleaning chemicals and sanitizers, and comprehensive documentation supporting food safety management systems.

FDA Material Compliance Requirements
21 CFR 177.26002 – Rubber Articles Intended for Repeated Use:
- Materials must be listed in FDA regulations
- Migration testing required for food contact surfaces
- Temperature and pH limitations must be observed
- Extraction testing with food simulants
Approved Material Categories:
- Stainless steel: 316L minimum for food contact
- Food grade elastomers: EPDM, FKM, silicone with FDA approval
- Engineering plastics: PEEK, POM, PTFE with proper certification
- Coatings and lubricants: NSF H1 approved only
Material Documentation Requirements:
- Certificate of compliance from material supplier
- Migration test reports for food contact applications
- Temperature and chemical resistance data
- Traceability records for material lots
Hassan recently told me: “Chuck, your detailed material compliance documentation saved us during our USDA inspection. The inspector was impressed with the completeness of our records.”
NSF Certification Standards
NSF/ANSI 51 – Food Equipment Materials:
- Covers materials in direct and indirect food contact
- Requires independent testing and certification
- Annual surveillance audits for continued compliance
- Public database of certified products
NSF/ANSI 2 – Food Equipment Design:
- Sanitary design requirements
- Cleanability and inspectability standards
- Drainage and accessibility requirements
- Material junction specifications
Regulatory Zone Classifications
Zone 1 – Direct Food Contact:
- Surfaces directly touching food products
- Strictest material and design requirements
- Regular cleaning and sanitization protocols
- FDA 21 CFR 177 compliance mandatory
Zone 2 – Food Splash Zone:
- Areas subject to food splashing or aerosols
- Food grade materials recommended
- Enhanced cleaning procedures required
- NSF certification preferred
Zone 3 – Non-Food Areas:
- Equipment rooms and utility areas
- Standard industrial materials acceptable
- Basic sanitation requirements
- Focus on preventing contamination migration
At Bepto, we maintain comprehensive food grade certifications and can provide detailed compliance documentation for any food processing application. Our materials team stays current with all regulatory updates. 😉
How Do FDA and NSF Standards Apply to Cable Gland Materials and Design?
FDA and NSF standards establish specific requirements for materials, surface finishes, and design features that affect food safety. These standards directly impact cable gland selection and specification.
FDA and NSF standards require non-toxic materials with documented food contact approval, smooth surfaces without harboring bacteria, chemical resistance to cleaning agents, temperature stability under processing conditions, and design features that prevent contamination accumulation.
Material Selection Criteria
Stainless Steel Requirements:
- Grade 316L minimum: Superior corrosion resistance
- Surface finish: 32 Ra or better for food contact
- Passivation3: ASTM A967 compliant surface treatment
- Welding: Proper heat treatment to maintain corrosion resistance
Elastomer Selection:
- EPDM: Excellent for dairy and beverage applications
- FKM (Viton): High temperature and chemical resistance
- Silicone: Wide temperature range, FDA approved grades
- Avoid: NBR, natural rubber, and non-approved compounds
Engineering Plastic Options:
- PEEK: Exceptional chemical and temperature resistance
- POM (Delrin): Good mechanical properties, limited temperature
- PTFE: Excellent chemical resistance, low friction
- Nylon: Limited use due to moisture absorption
Surface Finish Requirements
Critical Surface Characteristics:
- Roughness: Ra ≤ 32 microinches for direct food contact
- Porosity: Non-porous surfaces prevent bacterial growth
- Crevices: Eliminate or minimize to prevent contamination
- Drainage: Design for complete liquid removal
David shared: “The surface finish requirements were more stringent than we expected. Your guidance on proper specifications prevented costly rework.”
Chemical Resistance Testing
Cleaning Agent Compatibility:
- Alkaline cleaners: Sodium hydroxide solutions up to 4%
- Acid cleaners: Nitric and phosphoric acid solutions
- Sanitizers: Chlorine, quaternary ammonium4, peracetic acid
- CIP solutions: Combined cleaning and sanitizing chemicals
Testing Protocols:
- ASTM D543 – Chemical resistance of plastics
- ASTM D471 – Rubber property changes in liquids
- FDA extraction testing with food simulants
- Accelerated aging under processing conditions
Temperature Performance Requirements
Processing Temperature Ranges:
- Pasteurization: 72-85°C continuous exposure
- Sterilization: 121-134°C for specified durations
- Cleaning cycles: 85-95°C with chemical solutions
- Thermal cycling: Multiple heating and cooling cycles
Material Performance Validation:
- Long-term stability testing at maximum temperatures
- Thermal cycling to simulate process conditions
- Property retention after cleaning chemical exposure
- Seal integrity under temperature variations
What Sanitary Design Principles Must Cable Glands Follow in Food Processing?
Sanitary design principles ensure cable glands don’t create contamination risks or cleaning challenges in food processing environments. These principles are fundamental to food safety compliance.
Sanitary design principles require smooth, continuous surfaces without crevices, self-draining configurations, accessibility for cleaning and inspection, materials that withstand cleaning chemicals, and design features that prevent bacterial growth and contamination accumulation.
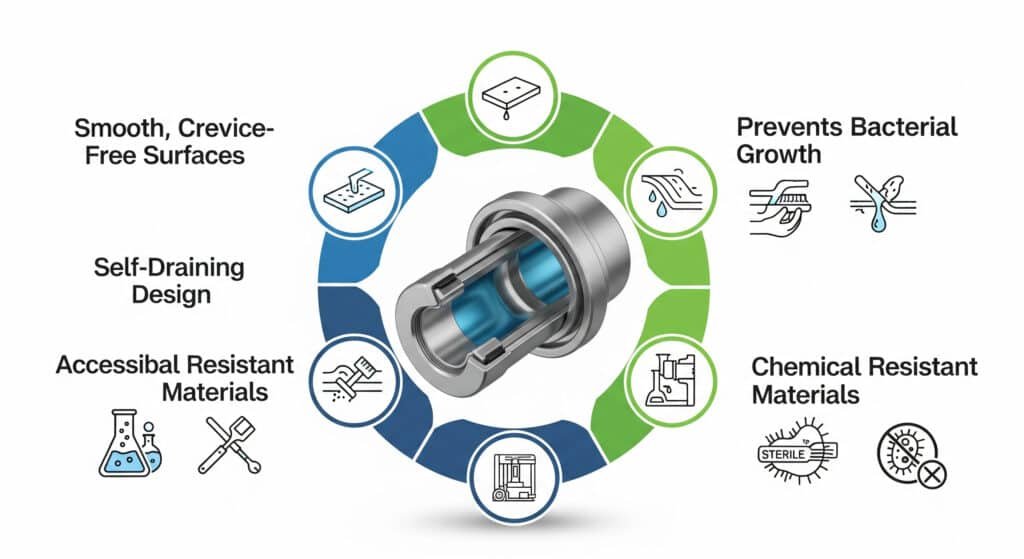
Surface Design Requirements
Smooth Surface Principles:
- No sharp corners: Minimum 3mm radius on all edges
- Continuous surfaces: Avoid joints and seams where possible
- Polished finishes: 32 Ra or better for direct food contact
- Weld quality: Smooth, continuous welds without undercuts
Crevice Elimination:
- Gasket design: Captured seals prevent contamination traps
- Thread design: Minimize exposed threads in food zones
- Component interfaces: Flush mounting without gaps
- Cable entry: Sealed against ingress of cleaning solutions
Drainage and Accessibility
Self-Draining Design:
- Slope requirements: Minimum 1:100 slope for liquid drainage
- Low points: Eliminate areas where liquids can pool
- Orientation: Design for proper installation orientation
- Drain paths: Clear, unobstructed drainage routes
Cleaning Accessibility:
- Visual inspection: All surfaces must be visible or accessible
- Cleaning reach: Within standard cleaning tool reach
- Disassembly: Designed for easy cleaning access when required
- Tool requirements: Standard tools for maintenance access
Hassan told me: “The sanitary design principles you taught us transformed our approach to equipment specification. Our cleaning efficiency improved dramatically.”
Hygienic Connection Methods
Sanitary Clamp Connections:
- Tri-clamp fittings: Standard sanitary connections
- Gasket materials: FDA-approved elastomers only
- Surface finish: Consistent with piping system requirements
- Installation: Proper alignment and clamping force
Threaded Connections:
- Thread design: Minimize exposed threads
- Sealant materials: NSF approved thread sealants only
- Orientation: Threads positioned to avoid contamination
- Maintenance: Easy disassembly for cleaning
Material Junction Requirements
Dissimilar Material Interfaces:
- Galvanic compatibility: Prevent corrosion at interfaces
- Thermal expansion: Account for different expansion rates
- Seal design: Maintain integrity across material changes
- Documentation: Material compatibility verification
Fastener Requirements:
- Stainless steel: 316L minimum for food zones
- Head design: Smooth, cleanable fastener heads
- Thread treatment: Anti-seize compounds must be food grade
- Torque specifications: Proper sealing without over-stress
At Bepto, our sanitary design expertise ensures all food grade cable glands meet or exceed industry sanitary design standards. We provide detailed installation guidelines to maintain sanitary integrity. 😉
How Do You Specify and Verify Food Grade Cable Glands for Different Processing Zones?
Proper specification and verification of food grade cable glands requires understanding zone-specific requirements, material compatibility, and documentation needs for regulatory compliance.
Food grade cable gland specification requires zone classification analysis, material selection based on food contact requirements, cleaning protocol compatibility, temperature and chemical resistance verification, and comprehensive documentation supporting HACCP and regulatory compliance programs.
Zone-Specific Specification Requirements
Zone 1 (Direct Food Contact) Requirements:
- FDA 21 CFR 177 compliant materials mandatory
- NSF certification for all components
- Surface finish Ra ≤ 32 microinches
- Complete drainage capability
- CIP (Clean-in-Place)5 compatibility
Zone 2 (Food Splash Zone) Requirements:
- Food grade materials strongly recommended
- Chemical resistance to cleaning agents
- Sanitary design principles applied
- Regular cleaning protocol compatibility
- Inspection accessibility maintained
Zone 3 (Non-Food Areas) Requirements:
- Standard industrial materials acceptable
- Focus on preventing contamination migration
- Basic chemical resistance requirements
- Maintenance accessibility considerations
- Cost optimization opportunities
Specification Development Process
Step 1: Application Analysis
- Identify processing zone classification
- Determine food contact probability
- Analyze cleaning and sanitization requirements
- Evaluate temperature and chemical exposure
- Assess maintenance and inspection needs
Step 2: Material Selection
- Select FDA-compliant base materials
- Choose appropriate elastomer compounds
- Verify chemical compatibility with process
- Confirm temperature performance requirements
- Validate cleaning agent resistance
Step 3: Design Verification
- Apply sanitary design principles
- Ensure drainage and accessibility
- Minimize contamination risk areas
- Verify cleaning protocol compatibility
- Confirm installation requirements
David recently shared: “Your systematic specification process helped us avoid over-specifying in non-critical areas while ensuring full compliance where it mattered.”
Documentation and Verification Requirements
Essential Documentation Package:
- Material certificates of compliance
- FDA regulation compliance statements
- NSF certification documents
- Chemical resistance test reports
- Temperature performance validation
- Cleaning protocol compatibility verification
Verification Procedures:
- Incoming inspection: Material and marking verification
- Installation verification: Proper orientation and sealing
- Performance testing: Leak testing and function verification
- Cleaning validation: Effectiveness of cleaning procedures
- Periodic inspection: Ongoing compliance monitoring
HACCP Integration Requirements
Hazard Analysis Integration:
- Include cable glands in hazard analysis
- Identify potential contamination sources
- Establish critical control points where applicable
- Define monitoring procedures
- Establish corrective action protocols
Documentation Requirements:
- Supplier qualification records
- Material traceability documentation
- Installation and maintenance procedures
- Cleaning and sanitization protocols
- Inspection and monitoring records
Hassan told me: “Integrating cable gland requirements into our HACCP system provided a comprehensive approach to contamination prevention.”
Supplier Qualification Criteria
Technical Qualifications:
- FDA and NSF certification capabilities
- Food industry experience and references
- Quality system certification (ISO 9001 minimum)
- Technical support and documentation capabilities
- Regulatory compliance tracking and updates
Business Qualifications:
- Financial stability for long-term support
- Global supply chain capabilities
- Inventory management and availability
- Change control and notification procedures
- Emergency response and support capabilities
Common Specification Mistakes
Technical Errors:
- Under-specifying zone requirements
- Ignoring cleaning chemical compatibility
- Inadequate temperature performance margins
- Missing drainage and accessibility requirements
- Insufficient documentation requirements
Commercial Mistakes:
- Over-specifying non-critical applications
- Ignoring total cost of ownership
- Inadequate supplier qualification
- Missing spare parts and service requirements
- Poor change control procedures
Bepto’s Food Grade Support Services
We provide comprehensive support for food processing applications:
- Complete certification packages for FDA and NSF compliance
- Application engineering for zone-specific requirements
- Cleaning validation support and documentation
- HACCP integration assistance and training
- Regulatory update notifications and compliance guidance
David recently said: “Working with Bepto’s food grade team gave us confidence that our specifications were both compliant and cost-effective. Their industry expertise was invaluable.”
Conclusion
Food grade compliance for cable glands requires systematic attention to materials, design, documentation, and zone-specific requirements to ensure food safety and regulatory compliance.
FAQs About Food Grade Cable Glands
Q: Do all cable glands in a food processing plant need to be food grade?
A: No, only cable glands in zones with potential food contact or contamination risk require food grade materials. Zone 3 areas can use standard industrial products, but proper zone classification analysis is essential to make this determination correctly.
Q: What’s the difference between FDA approval and NSF certification for cable glands?
A: FDA approval refers to materials meeting FDA regulations (21 CFR 177), while NSF certification involves independent testing and ongoing surveillance of complete products. NSF certification is more comprehensive and preferred for direct food contact applications.
Q: How often should food grade cable glands be replaced or inspected?
A: Inspection frequency depends on your HACCP plan and processing conditions, typically ranging from monthly to annually. Replacement depends on material degradation, cleaning chemical exposure, and performance requirements. At Bepto, we provide guidance on inspection criteria and replacement intervals.
Q: Can I use standard stainless steel cable glands in food processing applications?
A: Standard stainless steel may not meet food grade requirements due to surface finish, material grade, or design features. Food grade applications require 316L stainless steel with proper surface finish (Ra ≤ 32) and sanitary design principles.
Q: What documentation do I need for FDA inspections regarding cable glands?
A: You need material certificates showing FDA compliance, supplier qualification records, installation and maintenance procedures, cleaning validation documentation, and traceability records. At Bepto, we provide comprehensive documentation packages to support regulatory inspections.
-
Review the official FDA principles for Hazard Analysis and Critical Control Point (HACCP) systems. ↩
-
Access the full text of the FDA regulation 21 CFR 177.2600 for rubber articles in contact with food. ↩
-
Learn about the process of passivation and how it improves the corrosion resistance of stainless steel surfaces. ↩
-
Understand the properties and applications of Quaternary Ammonium Compounds (Quats) as sanitizers in the food industry. ↩
-
Explore the fundamental design principles and operational benefits of Clean-in-Place (CIP) systems. ↩



