Corrosion failures in cable glands cause catastrophic system downtime, safety hazards, and millions in replacement costs that could be prevented through proper understanding of electrochemical processes and material selection. Engineers often underestimate corrosion mechanisms, leading to premature failures in marine, chemical, and industrial environments where aggressive conditions accelerate material degradation. Poor material choices result in galvanic corrosion, stress corrosion cracking, and environmental attack that compromises both electrical and mechanical integrity.
Understanding corrosion chemistry reveals that material selection must consider galvanic compatibility, environmental exposure conditions, and electrochemical potential differences, with proper alloy selection and surface treatments providing 10-50 times longer service life in corrosive environments. Comprehensive corrosion analysis ensures optimal material choice for maximum longevity.
After analyzing corrosion failures from over 5,000 cable gland installations across marine, chemical processing, and offshore applications, I’ve identified the critical electrochemical factors that determine material performance and longevity. Let me share the comprehensive corrosion science that will guide your material selection and ensure exceptional durability in the most aggressive environments.
Table of Contents
- Understanding the Fundamental Chemistry of Corrosion in Cable Glands
- How Different Materials Respond to Corrosive Environments
- Galvanic Corrosion: The Hidden Threat in Multi-Material Systems
- Advanced Surface Treatments and Protective Coatings
- FAQs About Corrosion Prevention in Cable Gland Applications
Understanding the Fundamental Chemistry of Corrosion in Cable Glands
Corrosion is fundamentally an electrochemical process1 where metals lose electrons and revert to their natural oxidized state, with the rate and mechanism dependent on material properties and environmental conditions.
Corrosion occurs when metals act as anodes in electrochemical cells, losing electrons to form metal ions while oxygen or other oxidizers accept electrons at cathode sites, with the process accelerated by electrolytes, temperature, and pH conditions commonly found in industrial environments. Understanding these mechanisms enables effective prevention strategies.
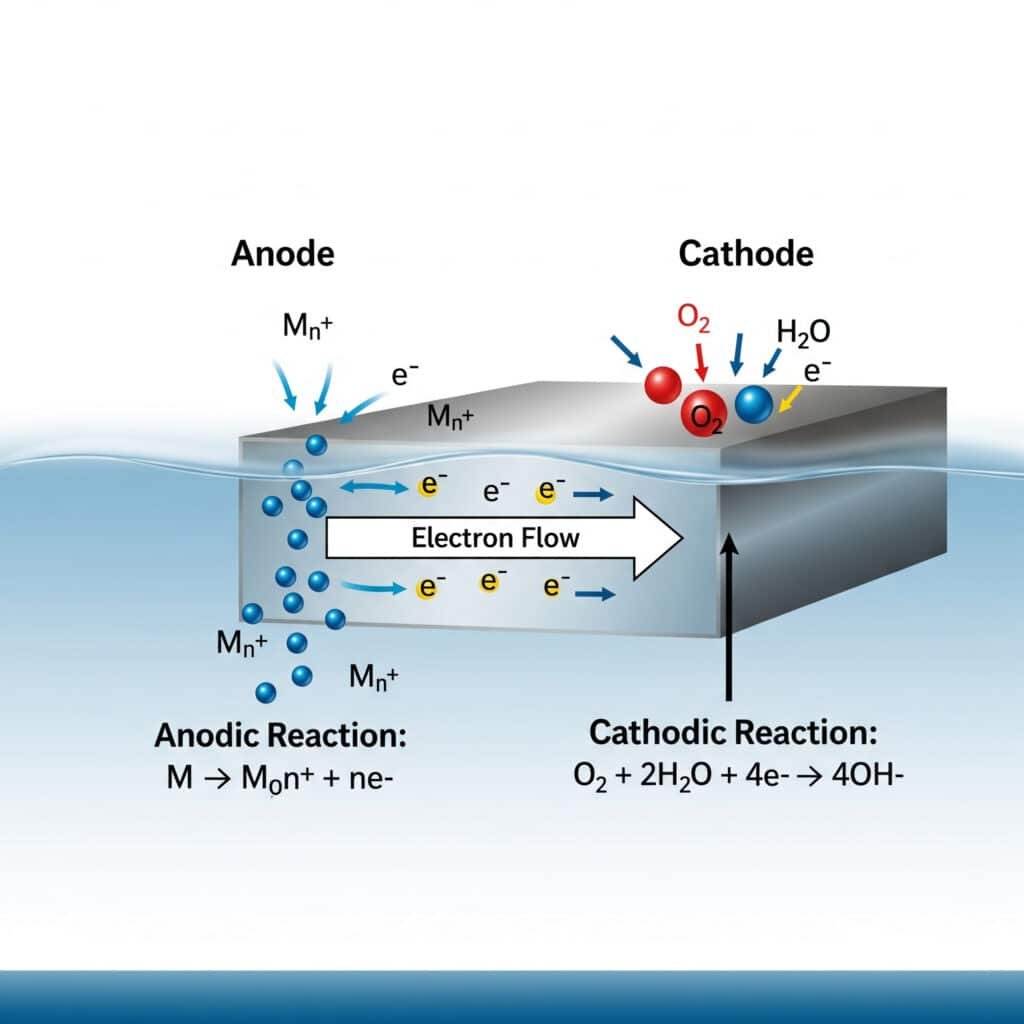
Electrochemical Fundamentals
Basic Corrosion Reactions:
- Anodic reaction: M → M^n+ + ne^- (metal oxidation)
- Cathodic reaction: O₂ + 4H^+ + 4e^- → 2H₂O (oxygen reduction, acidic)
- Cathodic reaction: O₂ + 2H₂O + 4e^- → 4OH^- (oxygen reduction, alkaline)
- Overall process: Metal dissolution coupled with electron consumption
Thermodynamic Driving Forces:
- Standard electrode potentials: Determine corrosion tendency
- Galvanic series2: Practical nobility ranking in seawater
- Pourbaix diagrams3: pH and potential stability relationships
- Free energy changes: Thermodynamic favorability of corrosion reactions
Environmental Factors Affecting Corrosion
Electrolyte Composition:
- Chloride concentration: Aggressive anion that breaks down passive films
- pH levels: Affects metal stability and corrosion product formation
- Dissolved oxygen: Primary cathodic reactant in neutral/alkaline conditions
- Temperature: Accelerates reaction kinetics (2x rate per 10°C increase)
- Conductivity: Higher ionic strength increases corrosion current
Physical Environmental Factors:
- Moisture levels: Required for electrochemical reactions
- Temperature cycling: Thermal stress affects protective films
- UV exposure: Degrades organic coatings and polymers
- Mechanical stress: Accelerates corrosion through stress concentration
- Crevice conditions: Differential aeration creates aggressive local environments
Working with David, a maintenance engineer at a major petrochemical facility in Texas, we investigated cable gland failures in their sulfur processing units. Hydrogen sulfide exposure was causing rapid corrosion of standard stainless steel glands. Our corrosion analysis revealed that upgrading to super duplex stainless steel (UNS S32750) eliminated failures and extended service life from 2 years to 15+ years.
Corrosion Mechanisms in Cable Glands
Uniform Corrosion:
- Mechanism: Even metal loss across exposed surfaces
- Rate factors: Material composition, environment aggressiveness
- Predictability: Relatively predictable based on corrosion rate data
- Prevention: Proper material selection, protective coatings
Localized Corrosion:
- Pitting corrosion: Concentrated attack creating deep penetrations
- Crevice corrosion: Aggressive conditions in confined spaces
- Stress corrosion cracking4: Combined stress and corrosive environment
- Intergranular corrosion: Attack along grain boundaries in sensitized alloys
Material-Specific Corrosion Behavior
| Material | Primary Corrosion Modes | Critical Environments | Protective Mechanisms |
|---|---|---|---|
| Carbon Steel | Uniform, pitting | Marine, acidic | Coatings, cathodic protection |
| Stainless Steel 316 | Pitting, crevice | Chloride solutions | Passive film, proper grade selection |
| Aluminum Alloys | Pitting, galvanic | Marine, alkaline | Anodizing, alloy selection |
| Brass | Dezincification, SCC | Ammonia, stress | Inhibited alloys, stress relief |
| Inconel 625 | Minimal corrosion | Extreme environments | Chromium oxide film |
How Different Materials Respond to Corrosive Environments
Material selection must consider specific corrosion mechanisms and environmental conditions to ensure optimal performance and longevity.
Different materials exhibit vastly different corrosion resistance based on their chemical composition, microstructure, and ability to form protective surface films, with stainless steels relying on chromium oxide passivity, aluminum forming protective oxide layers, and specialty alloys using multiple alloying elements for enhanced protection. Understanding material-environment interactions guides optimal selection.
Stainless Steel Performance Analysis
Austenitic Stainless Steels (300 Series):
- 316L composition: 17-20% Cr, 10-14% Ni, 2-3% Mo, <0.03% C
- Corrosion resistance: Excellent in most environments, limited in high-chloride
- Pitting resistance: PREN = %Cr + 3.3(%Mo) + 16(%N) ≈ 25-27
- Critical applications: Marine, food processing, chemical handling
- Limitations: Chloride-induced pitting above 60°C, stress corrosion cracking
Duplex Stainless Steels:
- 2205 composition: 22% Cr, 5% Ni, 3% Mo, balanced ferrite/austenite
- Corrosion resistance: Superior to 316L, excellent chloride resistance
- Pitting resistance: PREN ≈ 35, significantly higher than austenitic grades
- Mechanical properties: Higher strength, better stress corrosion resistance
- Applications: Offshore, chemical processing, high-chloride environments
Super Duplex Stainless Steels:
- 2507 composition: 25% Cr, 7% Ni, 4% Mo, nitrogen addition
- Corrosion resistance: Exceptional performance in aggressive environments
- Pitting resistance: PREN ≈ 42, suitable for severe service
- Cost considerations: 3-5x cost of 316L, justified for critical applications
- Applications: Seawater systems, chemical processing, offshore platforms
Working with Hassan, who manages corrosion control for a major desalination plant in Saudi Arabia, we evaluated cable gland performance in high-temperature seawater environments. Standard 316L stainless steel showed pitting failures within 6 months. Our super duplex 2507 cable glands have operated for 5+ years without any corrosion issues, despite the aggressive 80°C seawater exposure.
Aluminum Alloy Corrosion Characteristics
6061-T6 Aluminum:
- Composition: 1% Mg, 0.6% Si, balanced aluminum
- Corrosion mechanism: Protective aluminum oxide film (Al₂O₃)
- Environmental sensitivity: Susceptible to pitting in chloride solutions
- Galvanic concerns: Anodic to most metals, requires isolation
- Applications: Aerospace, automotive, general industrial (non-marine)
5083 Marine Grade Aluminum:
- Composition: 4.5% Mg, enhanced corrosion resistance
- Corrosion resistance: Superior performance in marine environments
- Stress corrosion: Resistant to SCC in marine applications
- Welding considerations: Maintains corrosion resistance after welding
- Applications: Marine structures, offshore equipment, shipbuilding
Anodized Aluminum Performance:
- Type II anodizing: 10-25 μm oxide layer, enhanced corrosion protection
- Type III anodizing: 25-100 μm hard coat, superior durability
- Sealing treatments: Improve corrosion resistance in aggressive environments
- Performance improvement: 5-10x longer life vs. bare aluminum
- Limitations: Coating damage exposes substrate to accelerated corrosion
Specialty Alloy Performance
Inconel 625 (UNS N06625):
- Composition: 58% Ni, 20-23% Cr, 8-10% Mo, 3.6% Nb
- Corrosion resistance: Exceptional performance in extreme environments
- Temperature capability: Maintains properties to 650°C
- Chemical resistance: Resistant to acids, alkalis, oxidizing conditions
- Cost factor: 10-15x cost of stainless steel, justified for critical service
Hastelloy C-276:
- Composition: 57% Ni, 16% Cr, 16% Mo, 4% W
- Corrosion resistance: Superior performance in reducing acids
- Versatility: Excellent in both oxidizing and reducing environments
- Applications: Chemical processing, pollution control, waste treatment
- Performance: Virtually immune to stress corrosion cracking
Galvanic Corrosion: The Hidden Threat in Multi-Material Systems
Galvanic corrosion occurs when dissimilar metals are electrically connected in the presence of an electrolyte, creating accelerated corrosion of the more active metal.
Galvanic corrosion can increase corrosion rates by 10-100 times normal levels when incompatible metals are coupled, with the severity depending on the potential difference between materials, area ratios, and electrolyte conductivity, making material compatibility analysis critical for cable gland system design. Proper material selection prevents catastrophic galvanic failures.
Galvanic Series and Compatibility
Galvanic Series in Seawater (most to least noble):
- Platinum, Gold – Highly cathodic (protected)
- Inconel 625, Hastelloy C – Excellent nobility
- 316 Stainless Steel (passive) – Good nobility when passive
- Copper, Bronze – Moderate nobility
- Brass – Moderate activity
- Carbon Steel – Active (corrodes readily)
- Aluminum Alloys – Highly active
- Zinc – Most active (sacrificial)
Compatibility Guidelines:
- Safe combinations: Materials within 0.25V potential difference
- Caution zone: 0.25-0.50V difference, requires evaluation
- Dangerous combinations: >0.50V difference, avoid direct contact
- Area effects: Large cathode/small anode ratios accelerate corrosion
- Distance effects: Galvanic current decreases with separation distance
Real-World Galvanic Corrosion Examples
Case Study 1: Aluminum Cable Glands with Steel Enclosures
- Problem: Aluminum glands corroding rapidly when mounted to steel panels
- Mechanism: Aluminum anodic to steel, accelerated dissolution
- Solution: Stainless steel isolation washers, dielectric coatings
- Result: Extended service life from 6 months to 5+ years
Case Study 2: Brass Glands with Aluminum Cables
- Problem: Aluminum cable lugs corroding at brass gland interface
- Mechanism: Aluminum anodic to brass, concentrated attack at connection
- Solution: Tin-plated aluminum lugs, anti-corrosion compounds
- Result: Eliminated galvanic corrosion, maintained electrical integrity
Working with Maria, a corrosion engineer at a major offshore wind farm operator, we addressed galvanic corrosion between aluminum cable glands and steel tower structures. The original design showed severe aluminum corrosion within 18 months. Our solution using 316L stainless steel glands with proper isolation eliminated galvanic effects and achieved 25-year design life.
Galvanic Corrosion Prevention Strategies
Material Selection Approaches:
- Compatible materials: Use metals close in galvanic series
- Sacrificial protection: Deliberately use more active materials as anodes
- Noble material systems: Use corrosion-resistant alloys throughout
- Coating systems: Isolate dissimilar metals with protective barriers
Design Solutions:
- Electrical isolation: Non-conductive gaskets, bushings, coatings
- Area ratio optimization: Minimize anode area relative to cathode
- Drainage design: Prevent electrolyte accumulation in crevices
- Accessibility: Design for inspection and maintenance access
Environmental Factors Affecting Galvanic Corrosion
| Environment | Electrolyte Conductivity | Galvanic Risk | Prevention Priority |
|---|---|---|---|
| Marine/Seawater | Very High | Extreme | Critical – use compatible materials |
| Industrial/Chemical | High | Severe | Important – isolation required |
| Urban/Polluted | Moderate | Moderate | Recommended – protective measures |
| Rural/Dry | Low | Minimal | Basic – standard practices adequate |
Advanced Surface Treatments and Protective Coatings
Surface treatments and coatings provide additional corrosion protection beyond base material selection, often extending service life by 5-20 times.
Advanced surface treatments including electroplating, conversion coatings, and organic systems create barrier protection and modify surface electrochemistry to prevent corrosion initiation, with proper selection and application providing decades of protection in aggressive environments. Understanding coating mechanisms ensures optimal protection strategies.
Electroplating Systems
Zinc Plating:
- Mechanism: Sacrificial protection of steel substrates
- Thickness: 5-25 μm typical, thicker for severe service
- Performance: 1-5 years protection depending on environment
- Applications: General industrial, moderate corrosion environments
- Limitations: Limited temperature capability (<100°C)
Nickel Plating:
- Mechanism: Barrier protection with excellent corrosion resistance
- Thickness: 10-50 μm for corrosion protection
- Performance: 10-20 years in moderate environments
- Applications: Marine, chemical processing, decorative
- Advantages: Hard surface, wear resistance, temperature capability
Chromium Plating:
- Mechanism: Extremely hard, corrosion-resistant surface
- Types: Decorative (thin) vs. hard chrome (thick)
- Performance: Exceptional durability in aggressive environments
- Applications: Hydraulic systems, chemical processing, wear resistance
- Environmental concerns: Hexavalent chromium regulations
Conversion Coatings
Chromate Conversion (Aluminum):
- Mechanism: Chemical conversion of aluminum surface to chromate film
- Performance: Excellent corrosion protection and paint adhesion
- Thickness: 1-5 μm, transparent to golden color
- Applications: Aerospace, military, high-performance requirements
- Regulations: RoHS restrictions driving alternative treatments
Phosphate Conversion (Steel):
- Mechanism: Iron/zinc/manganese phosphate crystal formation
- Performance: Excellent base for paint systems, moderate standalone protection
- Applications: Automotive, appliance, general manufacturing
- Benefits: Improved paint adhesion, break-in lubrication
- Process: Acid cleaning, phosphating, neutralizing, drying
Anodizing (Aluminum):
- Type II: 10-25 μm, decorative and moderate protection
- Type III: 25-100 μm, hard coat for severe service
- Sealing: Improves corrosion resistance significantly
- Performance: 10-25 years in marine environments when properly sealed
- Applications: Architectural, marine, aerospace, electronics
Organic Coating Systems
Powder Coatings:
- Chemistry: Epoxy, polyester, polyurethane, hybrid systems
- Application: Electrostatic spray, thermal cure
- Performance: Excellent durability, chemical resistance
- Thickness: 50-150 μm typical
- Advantages: Environmental compliance, excellent finish quality
Liquid Paint Systems:
- Primers: Zinc-rich, epoxy, polyurethane for corrosion protection
- Topcoats: Polyurethane, fluoropolymer for weather resistance
- System design: Multiple coats for maximum protection
- Performance: 15-25 years with proper system design
- Applications: Marine, chemical, architectural, industrial
Working with our coating specialists at Bepto Connector, we developed a multi-layer protection system for cable glands in offshore applications: zinc-rich epoxy primer, intermediate epoxy coat, and fluoropolymer topcoat. This system provides 25+ year protection in marine environments, significantly outperforming single-layer coatings.
Coating Selection Criteria
Environmental Considerations:
- Chemical exposure: Acid, alkali, solvent resistance requirements
- Temperature range: Operating and peak temperature limits
- UV exposure: Outdoor applications require UV-stable systems
- Mechanical demands: Abrasion, impact, flexibility requirements
- Electrical properties: Conductivity vs. insulation requirements
Performance Requirements:
- Service life: 5-25 years depending on application criticality
- Maintenance access: Recoating feasibility and frequency
- Initial cost: Coating system cost vs. performance benefits
- Lifecycle cost: Total cost including maintenance and replacement
- Regulatory compliance: Environmental and safety regulations
Coating Quality Assurance
Surface Preparation Standards:
- SSPC/NACE standards[^5]: Surface cleanliness requirements
- Profile requirements: Surface roughness for adhesion
- Contamination control: Oil, salt, moisture removal
- Environmental conditions: Temperature, humidity during application
- Quality control: Inspection and testing protocols
Performance Testing:
- Salt spray testing: ASTM B117, accelerated corrosion evaluation
- Cyclic testing: ASTM D5894, realistic environmental simulation
- Adhesion testing: Cross-cut, pull-off testing for coating integrity
- Thickness measurement: Coating uniformity and specification compliance
- Field monitoring: Long-term performance validation
At Bepto Connector, we understand that corrosion prevention requires comprehensive understanding of electrochemical processes, material compatibility, and environmental factors. Our advanced material selection, surface treatments, and quality assurance programs ensure exceptional corrosion resistance and extended service life in the most aggressive environments.
Conclusion
Corrosion chemistry fundamentally determines cable gland longevity through electrochemical processes that can be controlled through proper material selection, galvanic compatibility analysis, and advanced surface treatments. Understanding these mechanisms enables engineers to specify cable glands that deliver 10-50 times longer service life in corrosive environments.
Success requires comprehensive analysis of environmental conditions, material compatibility, and protection strategies rather than relying solely on generic specifications. At Bepto Connector, our deep understanding of corrosion science and extensive field experience ensures you receive cable glands optimized for exceptional durability in your specific corrosive environment.
FAQs About Corrosion Prevention in Cable Gland Applications
Q: How do I determine which cable gland material is best for my corrosive environment?
A: Analyze your specific environment including temperature, pH, chemical exposure, and chloride levels, then consult galvanic series data and material compatibility charts. For marine environments, super duplex stainless steel or Inconel provides optimal performance, while chemical processing may require Hastelloy or other specialty alloys.
Q: What is galvanic corrosion and how can I prevent it in my cable gland installation?
A: Galvanic corrosion occurs when dissimilar metals are electrically connected in an electrolyte, causing accelerated corrosion of the more active metal. Prevent it by using compatible materials (within 0.25V potential difference), electrical isolation with non-conductive gaskets, or protective coatings to break the galvanic circuit.
Q: How much longer will proper material selection extend cable gland service life?
A: Proper material selection can extend service life by 10-50 times depending on the environment. For example, upgrading from carbon steel to super duplex stainless steel in seawater can increase life from 1-2 years to 25+ years, while advanced coatings can provide additional 5-20x improvement.
Q: Are surface treatments and coatings worth the additional cost for corrosion protection?
A: Yes, surface treatments typically cost 10-30% more initially but can extend service life by 5-20 times, providing excellent return on investment. For example, anodized aluminum costs 20% more than bare aluminum but lasts 10 times longer in marine environments, resulting in significant lifecycle cost savings.
Q: How can I verify that my cable glands will resist corrosion in my specific application?
A: Request corrosion test data specific to your environment, conduct pilot installations for field validation, specify materials with proven track records in similar applications, and consider accelerated corrosion testing (salt spray, cyclic testing) to validate performance before full deployment.
-
Learn the fundamentals of electrochemistry, the study of chemical reactions that cause electrons to move. ↩
-
Learn about the key industry standards from AMPP (formerly NACE/SSPC) for the proper preparation of surfaces before coating. ↩
-
Discover how these potential/pH diagrams are used to predict the thermodynamic stability and corrosion behavior of metals in aqueous solutions. ↩
-
Explore this failure mechanism, where a combination of tensile stress and a corrosive environment leads to cracking in susceptible materials. ↩



